About
I am a physical chemist using laser spectroscopy, mass spectrometry, and chemical simulations to build new pictures of how proteins and catalysts operate on the molecular scale. My recent research projects focus on:
- Ion binding processes in biological signaling
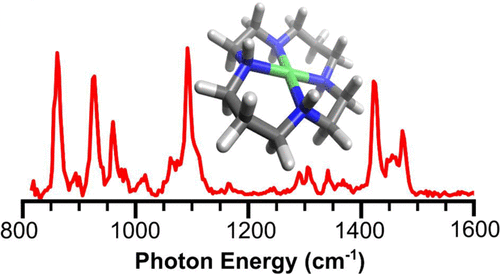
- Developing new experimental methods to probe the structure of reative chemical intermediates (such as those formed by the Ni-cyclam CO2 reduction catalyst)
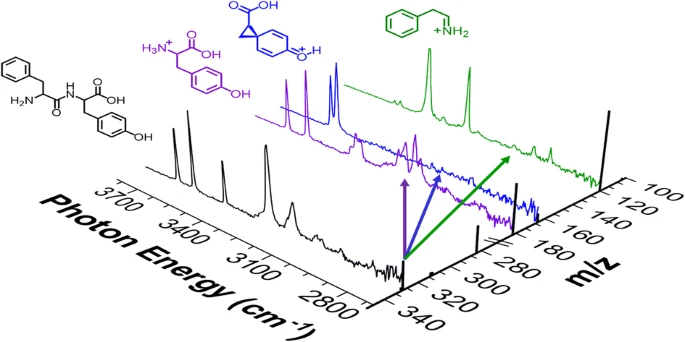
- The intrinsic spectral responses of the nitrile-containing vibrational reporters used to probe protein structure and dynamics.
I think making science accessible and welcoming to people from all backgrounds is key to an effective, innovative, and resilient research enterprise. But, more importantly, it's just the right thing to do. So, I am always working on making my teaching as inclusive as it can be, and on developing worthwhile outreach activities to inspire and encourage scientists-to-be.
Current Research
Broad research interests: physical chemistry, spectroscopy, biophysics, chemical dynamics, surface science
Ion binding in biological signaling
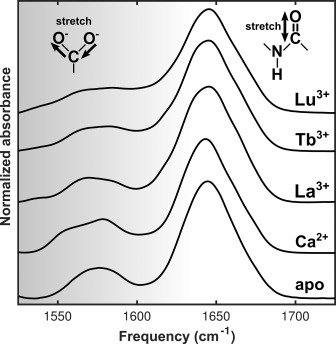
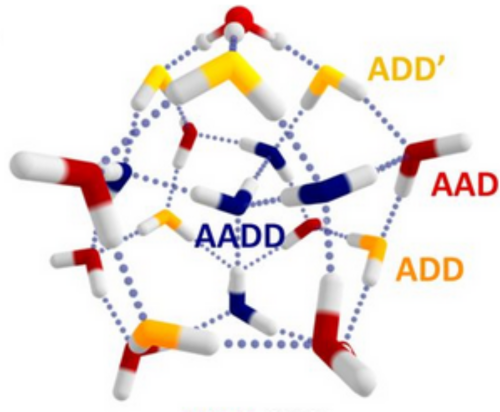
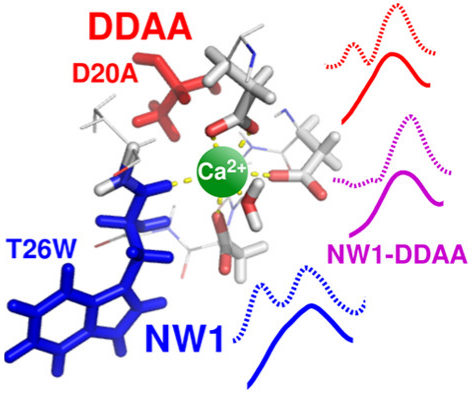
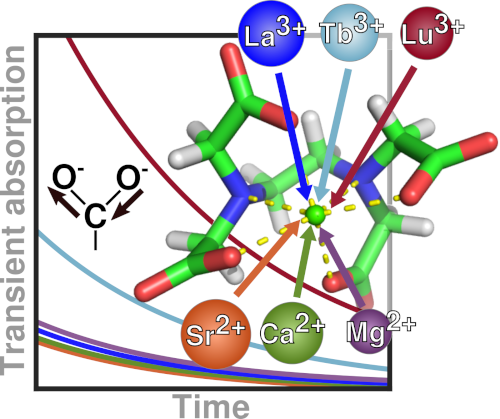
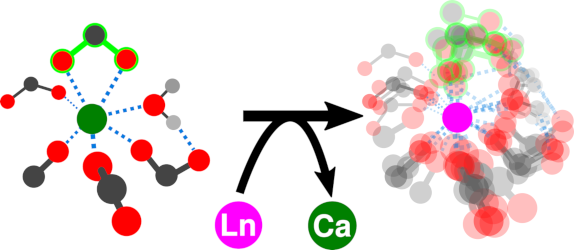
Signaling processes mediated by sensor proteins like those in the EF-hand family are essential to life in all eukaryotic cells. One particularly well-studied example, calmodulin, serves as a popular model for ion binding and activation in proteins. It transduces complex calcium signals and acts on hundreds of effector proteins, but the sensitivity and complexity of this process make it difficult to characterize. Much work uses lanthanides as luminescent calcium substitutes to study ion binding and activation in calmodulin and other proteins. This project exploits the time resolution and structural sensitivity of two-dimensional infarared spectroscopy to examine how calmodulin responds to binding with different divalent and trivalent ions and explores the ways in which substituting lanthanides for calcium indeed perturb calmodulin's binding site structure.
Experimental methods for probing intermediate structure in catalysis
A major challenge in chemistry is to study the structure and reactivity of intermediates formed in catalysis. These species often have short lifetimes and are too unstable to analyze using traditional techniques, such as X-ray diffraction or NMR spectroscopy. This project fuses inert atmosphere chemical reduction techniques, electrospray ionization (ESI) mass spectrometry, and cryogenic ion infrared spectroscopy into an experimental protocol capable of probing activated catalytic complexes.
Intrinsic responses of nitrile vibrational probes
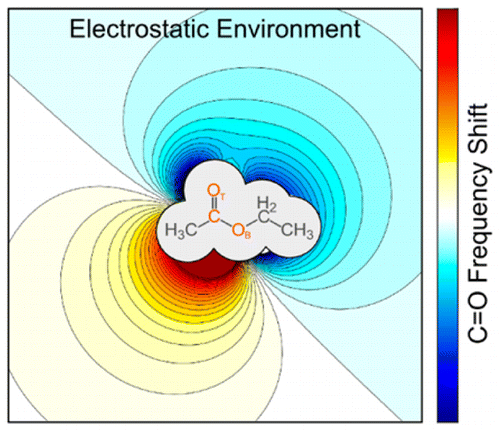
Spectroscopic reporters incorporating nitrile groups have emerged as a powerful tool for probing the structure and dynamics of proteins with the tools of infrared spectroscopy. However, solution-phase characterization of the probe response to its environment, encoded as the local electric field, is complicated by the response of the solvent. This project exploits cryogenic infrared predissocation spectroscopy to capture the spectral response of nitrile vibrational probes free from solvent effects.
 Sean C. Edington
Sean C. Edington